Metallic Iron as a Solution for Safe Drinking Water
Student submission by Karina Finlayson
Sustainable Development Goal (SDG) 6 targets the availability and management of water and sanitation. A key issue in achieving this goal is the availability of safe drinking water, particularly for rural communities and underdeveloped countries. The United Nations (UN) states that in order to reach the 2030 goals, there must be a drastic increase in the progress being made to meet drinking water, sanitation, and hygiene targets [1]. This need calls for new and innovative techniques to be introduced in order to combat water contamination globally.
The use of iron for water treatment has been investigated by chemists over the last three decades, and as such offers a well-researched solution for SDG 6. Previous solutions for safe drinking water have utilised iron as a decontaminant within a centralised plant. One such solution investigated the use of an iron wall within an aquifer, which allowed ground water to pass through. Over a five-year period, the water quality was tested, and it was conclusively found that the iron wall effectively worked to break down contaminants within the ground water [2]. A review of iron in a number of experiments utilising a similar set-up revealed that while the effectiveness of decontamination decreased over time, sites were able to effectively meet decontamination goals in this way [3].
When considering this solution for use in rural communities, it is necessary to consider the cost and logistics of implementing a large-scale solution within an aquifer. Additionally, such communities are often ill-equipped to deal with natural disasters which may impact a centralised solution such as this. As such, a de-centralised solution using metallic iron is proposed [4].
Metallic iron can be sourced locally within communities, and the decontamination process does not require any energy requirements or in depth chemistry knowledge [4]. This small-scale solution utilises gravity, sand, gravel, and rock alongside the metallic iron. As water passes through the system, it can be effectively treated similarly to passing water through an iron wall in an aquifer. This is shown in Figure 1, where the ‘influent’ represents contaminated water, and Fe0 represents metallic iron.
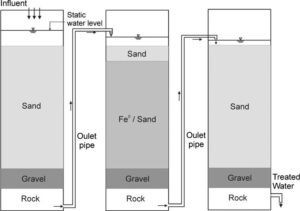
The primary concern raised in training communities on treating water with metallic iron is the potential for the iron to reach dangerous levels. As such, a detailed plan must be developed to ensure that communities are aware of methods for testing iron levels after undergoing the decontamination process.
By introducing the knowledge and basic systems required to treat ground water with metallic iron, safe drinking water can be made accessible to a larger number of communities globally. With an emphasis placed on the importance of testing the iron levels of the filtered water, the simple design of a metallic iron filter will allow communities to manage their water resources without any expertise in chemistry. This step forward in access to clean drinking water will aid in making global strides towards achieving SDG 6.
References
[1] United Nations Department of Economic and Social Affairs, “The Sustainable Development Goals Report 2022,” United Nations , 2022.
[2] S. F. O’Hannesin and R. W. Gillham, “Long-Term Performance of an In Situ “Iron Wall” for Remediation of VOCs,” Ground Water, vol. 36, no. 1, pp. 164-170, 1998.
[3] A. D. Henderson and A. H. Demond, “Long-Term Performance of Zero-Valent Iron Permeable,” Environmental Engineering Science, vol. 24, no. 4, pp. 401-423, 2007.
[4] C. Noubactep, “Metallic Iron for Water Treatment: A Critical Review,” Clean Soil Air Water, vol. 41, pp. 702-710, 2013.




